miraWave Technology.
The Sweat and Odar stops here
miraWave Technology
The Sweat and Odar stops here
miraWave Technology
In the axilla, eccrine and apocrine glands (the glands responsible for sweat and odor) and hair follicles are found in high density and varied depth at the dermal-fat interface. Peak temperatures in excess of 60°C throughout the entire volume of target tissue are required to achieve significant and permanent destruction of the glands. miraWave technology consistently delivers energy to the dermal-fat interface, allowing precise heating independent of skin thickness. With the miraDry® System, the energy is always delivered right where it is most effective.
No other technology provides the consistent density of energy delivery sufficient to destroy sweat and odor glands and hair to deliver superior results in as little as 1 treatment.
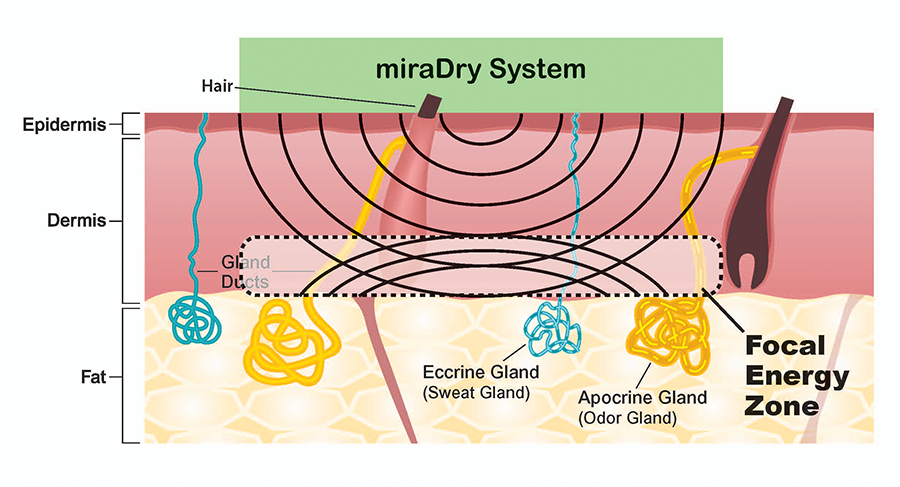
miraWave technology delivers 5.8 GHz microwave energy. The energy is reflected at the dermal-fat junction and becomes concentrated, creating a focal energy zone.
- Nearly all sweat and odor glands reside at the dermal-fat interface
- Focused heating is independent of skin thickness, hair color or skin color
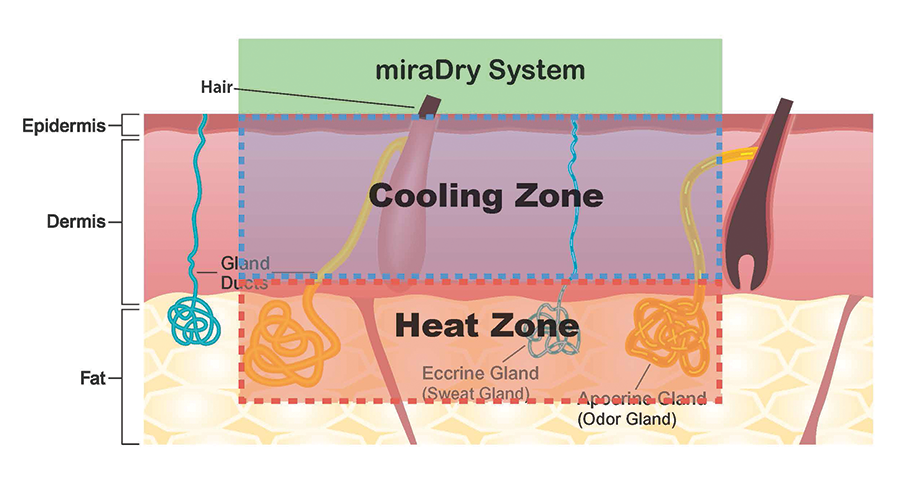
Continuous hydroceramic cooling protects the dermis while heat continues to spread down into the region where sweat glands reside
- Sweat and odor glands and hair follicles are destroyed in the heat zone
System Specification
| Microwave output frequency | • 5.8 GHz |
| Microwave energy output | • manufacturer defined set points (25% range) |
| Coolant | • Integrated cooling system uses deionized water at 15°C |
| Vacuum | • -508 to -559 mm of Hg (-20 to -22 inches of Hg) |
| Operating Voltage/Current | • 100-240V AC 50/60 Hz |
| Console Dimensions | • 1.19m H x 0.52m W x 0.81m D • 46.75” H x 20.25” W x 31.75” D With display folded down: • 0.90m H x 0.52m W x 0.81m D • 35.25” H x 20.25” W x 31.75” D |
| Console Weight | • 52.2 kg (112 lbs) |
| Conforms to | • 5 international standards |
Clinical Results
The miraDry Treatment has been proven, in multiple rigorously controlled studies and in over 70,000 commercial treatments, to deliver excellent efficacy, a strong safety profile, and high patient satisfaction.
Sweat
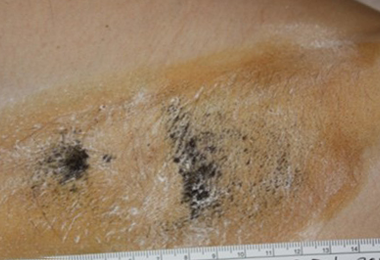
Patient A: partial treatment. Post-treatment starch iodine tests demonstrate dramatic and permanent sweat reduction.
Zone of Treatment
3 months
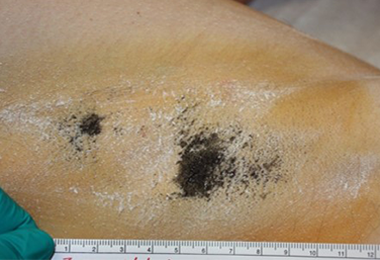
2 years
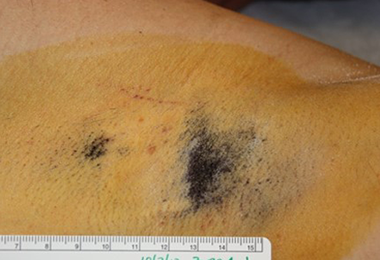
3 years
Patient is nearly sweat free in the area treated but continues to sweat in the area untreated.
Clinically Proven
Published studies demonstrate destruction and elimination of sweat and odor glands and permanent reduction of sweat.
Human histology illustrates sweat gland destruction.
Normal sweat glands
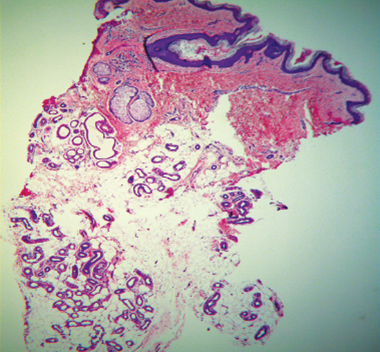
Baseline
Sweat gland cells devoid of nuclei – complete celluar necroisis
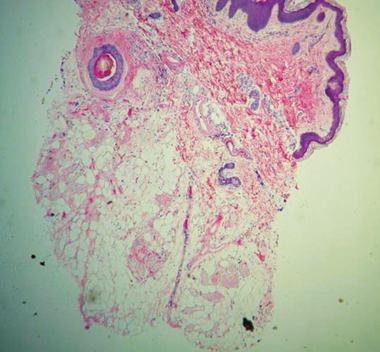
10 days POST-TREATMENT sample
No sweat glands
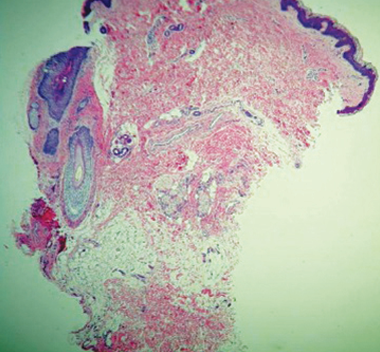
180 days POST-TREATMENT sample
“The ontogenesis of sweat glands is only at the embryonic period, so no new sweat glands are regenerated after birth.”1 Therefore, results are permanent.
Long-term studies demonstrate that miraDry® is effective for permanent reduction of sweat2.
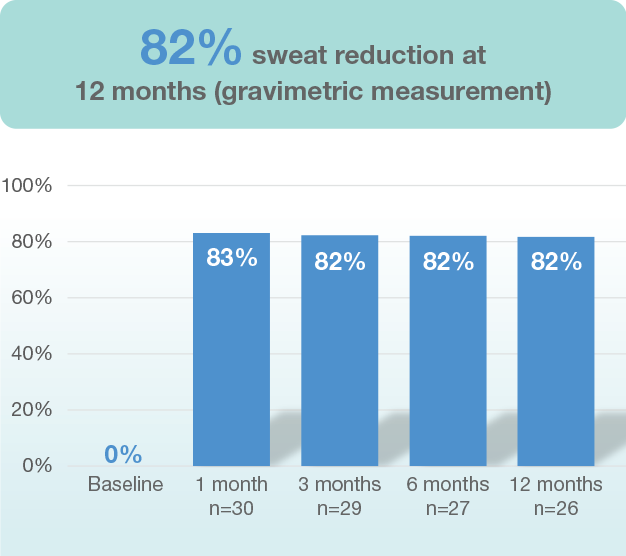
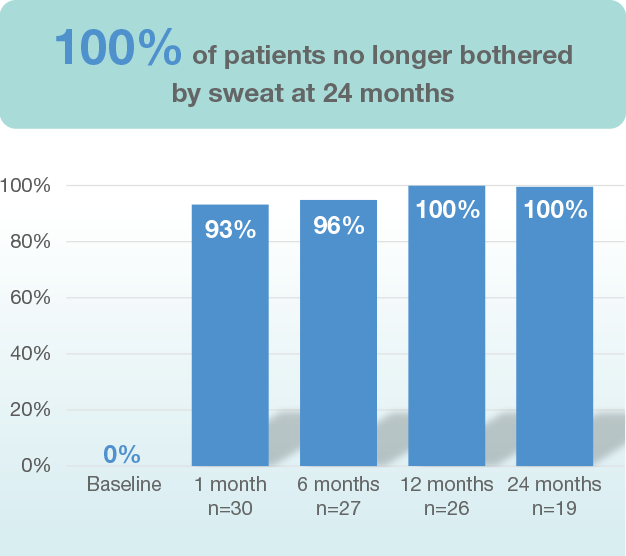
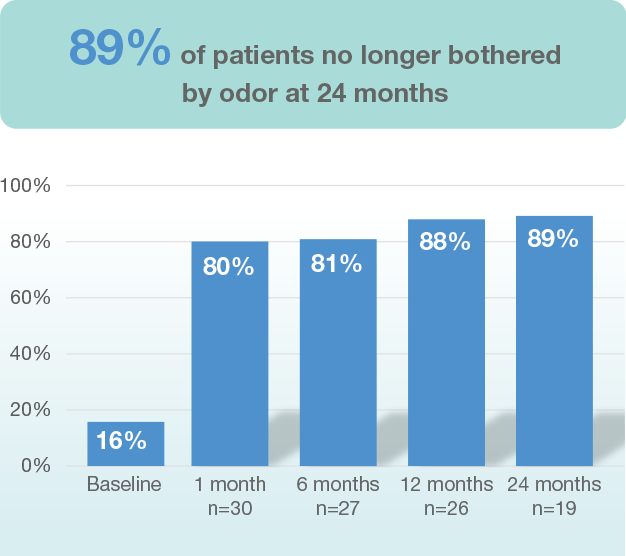
Hair
Long-term findings of approximately 70% reduction in underarm hair in patients in both light and dark axillary hair was seen, and is stable through 12 months of follow-up3.
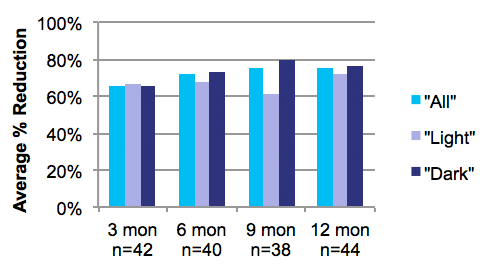
Average Hair Reduction from Quantitative Assessment for each follow-up visit. Results for light and dark hair are broken out separately for the initial 3m and final 12m visits.
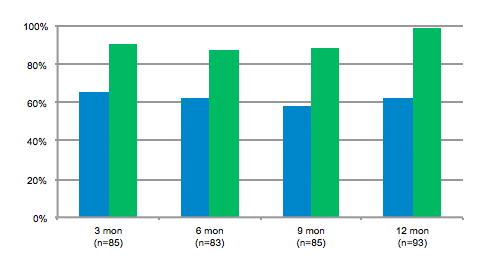
Results of side-by-side qualitative analysis per axilla. Percentage of axilla where the blinded reviewer correctly chose the baseline photo (green bars); % where assessor rated the reduction as being at least 50% (blue bars).
References: 1) Li H-H, Zhou G, Fu X-B, Zhang Lei. Antigen expression of human sweat glands. J Cutan Pathol 2009;36:318–24. 2) Long-term Efficacy and Quality of Life Assessment for Treatment of Axillary Hyperhidrosis With a Microwave Device. Mark Lupin, MD et al; Dermatol Surg 2014; 40: 805-807. 3) Long-term follow-up demonstrating reduction of axillary hair utilizing microwave technology by B. Zellickson et al. – abstract #60, presented at ASLMS 2015 Annual Meeting, April 2015 Kissimmee, FL.
Book Demo
I am interested in this equipment. Would like to book a demonstration of the same
Book Demoelōs hair removal can be done with an increased margin of safety. This is especially helpful in dark skinned patients. Creating the ultimate patient satisfaction safely Dr Kavitha Salem
Master Class Special Offer
Our Clients
Here are some of our esteemed clients.
















